Chronic myelocytic leukemia (CML)
Abstract:
CML belongs to the group of myeloproliferative neoplams and is characterized by a light to strong increase of mature granulocytes, myeloid precursor cells and platelets in the peripheral blood. At the molecular level, BCR-ABL-fusion gene is present. CML is a disease of the pluripotent bone marrow stem cell. BCR-ABL can be demonstrated in all myeloid cell lines and to a minor degree also in lymphocytes and endotelial cells. Untreated, the course of CML consists in a chronic phase of varying length, an accelerated phase and a terminal blastic phase. In 20 to 40% of the cases, CML is discovered by chance in asymptomatic patients.
The atypical CML (aCML, BCR/ABL-negative) is assigned to the myelodysplastic/myeloproliferative syndromes according to the WHO 2016 classification.
Clinical picture:
Symptoms of CML are night sweats, loss of wheight, abdominal discomfort due to splenomegaly or fatigue caused by anemia. The incidence is 1-2 cases per 100'000 per year. The median age is between 50 and 60 years. CML can occur at any age, but is rare in children and young adults. Triggering factors are not known.
Pathogenesis:
CML is caused by a balanced translocaltion between the chromosomes 9 and 22. The resulting fusion gene BCR-ABL codes for the fusion protein BCR-ABL . Contrary to the physiological tyrosinkinase ABL, whose expression is highly regulated, the aberrant BCR-ABL-tyrosinkinase is constitutively active and induces an uninhibited myeloid proliferation.
Therapy:
Until the year 2000, therapy of CML was based on hydroxyurea und alpha interferon. The response was not satifactory in most patients. Despite therapy, patients developed on average after 5 years an accelerated phase and a few months later a blast phase, which was almost always lethal.
The development of the oral tyrosinkinase-inhibitor Imatinib (Gleevec®) revolutionized the treatment of CML. Imitinib inhibits the constitutively active BCR-ABL-kinase and blocks the uncontrolled proliferation of CML-cells. This was the first example of targeted therapy that interrupts by means of a small-molecular inhibitor the pathogenetic mechanism of a disease. With imatinib, 97% of patients reached a complete hematological response. The 10-year survival rate is above 80%. CML-associated death has become rare. New ABL-inhibitors such as nilotionib (Tasigna@), dasatinib (Sprycel@), bosutinib (Bosulif@) oder ponatinib (Iclusig@) are more effective, sometimes even in imatinib-resistant cases. Allogenic stem cell transplantation has a place only in children and therapy refractory cases.
Hematology:
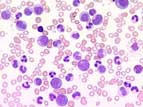
In the chronic phase of CML, often very high leukocyte counts are seen up to 300 x109/L . In the peripheral blood, all stages of development of the granulopoiesis from promyelocytes to segmented granulocytes are present. These are cytologically unobstrusive without signs of dyplasia. Blasts account for less than 2% in the chronic phase. Eosinophila is typical. The lack of basophils makes the diagnosis unlikely. Thrombocytosis is frequent. Anemia develops mostly with increasing leukocyte counts.
The accelerated phase is characterized by the persistence of leukocytosis, splenomegaly, thrombocytosis or thrombopenia despite therapy. Blasts can go up to 20%. In the blast phase, blasts account for more than 20% of nucleated cells in the peripheral blood.
Bone marrow:
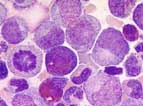
In the chronic phase, the bone marrow is hypercellular with clear predominance of the granulopoiesis. The different stages of maturation from promyelocytes to segmented granulocytes exist in normal proportions. Relevant disorders of maturation are not seen. Blasts account for less then 5% of all cells. The megakaryocytes are increased and smaller than normal, so called "dwarf like megakaryocytes". Typically, eosinophilia and basophilia are found. Pseudo-Gaucher-cells or "sea blue histiocytes" are typical but occur also in othe diseases. A reticulin fibrosis is found in 30% of the cases.
In the accelerated phase, blasts are increaed, but account for less than 20% of cells. The blast phase is an acute leukemia on the ground of a known CML. Blasts account for more than 20% of cells. 70% of the cases are of myeloid nature, often with monocytic, erythroid or megakaryocytic maturation. In 30% of cases, it is an acute lymphoblastic leukemia.
Diagnosis
The Diagnosis of CML can be established if simultaneously a BCR-ABL-fusion gene and mature granulopoietic cells in the blood and bone marrow are present.
A BCR-ABL-fusion gene can also be found in some cases of acute B-lymphoblastic leukemia (B-ALL) and sometimes in acute myelocytic leukemia (AML). In rare cases, a CML can manifest as isolated thrombocytosis. Therefore, BCR-ABL must be excluded by means of RT-PCR.
If CML is suspected, BCR-ABL mus be searched with RT-PCR. Additionally, the examination of the bone marrow including cytogenetics are obligatory elements of the initial diagnosis. Cytogenetically, a balanced tranlocation (9;22) can be found in 90-95% of the cases, the so called Philadelphia-Chromosom (Ph). Additional cytogenetic alterations give important prognostic information.